Manufacturer / Importer of Medical Devices
“manufacture” means an act of making, assembling, inventing, divisional repackaging, combinational re-packaging, improving, transforming, modifying or sterilizing
“import” means an act of bringing or ordering into the Kingdom
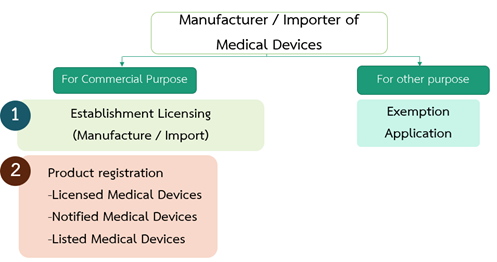
Manufacturer / Importer of Medical Devices for Commercial Purpose
- Establishment Licensing
Any person who wishes to manufacture or import medical devices shall carry out registration of an establishment with the permission grantor
- Product Registration
In order to regulate medical devices effectively, risk-based classification is additionally used to classify licensed, notified, and listed medical devices into 4 classes by evaluating risks associated with the devices. The risk-based classification is harmonized with the regional and international regulations to assure safety and effectiveness of the devices as well as to protect consumers and patients appropriately.
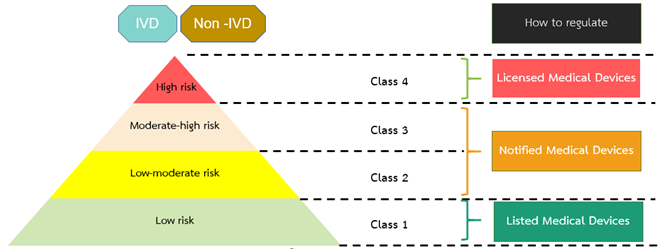
If you want to register a medical device, you must first submit a risk classification that complies with the ASEAN MEDICAL DEVICE DIRECTIVE Annex 2 (non-IVD) and Annex 3 (IVD) for your devices by submitting supporting documents as the table below
Medical Device Risk Classification | Risk level | Submission Dossier |
Class 1 and Animal Medical Devices | Low risk | |
Class 2 | Low-moderate risk | |
Class 3 | Moderate-high risk | |
Class 4 | High risk |
An establishment registrant, as a manufacturer or importer of medical devices, who wishes to manufacture or import medical devices shall submit an application for certificate of licensed or notified or listed medical device depending on risk classification.
The registrant shall carry out the manufacture or import when the licensor has issued the certificate of licensed or notified or listed medical device.
Manufacturer / Importer of Medical Devices for other purpose
Establishment licensing and product registration aforementioned shall not apply to:
(1) the manufacture, import or sale of medical devices by State agencies in the discharge of duties in connection with the prevention, diagnosis or treatment of diseases or the rehabilitation and the Thai Red Cross Society
(2) the manufacture of medical devices specifically for the purpose of sterilisation in medical establishments under the law on medical establishments
(3) the manufacture or sale of medical devices manufactured by medical and public health practitioners for their particularly individual patients or animals
(4) the sale of medical devices, in respect of which a licence, a specification declaration certificate or a notification certificate has been granted, by medical establishments or medical and public health practitioners for their particularly individual patients or animals;
(5) the manufacture or import of medical devices in the quantity meeting the necessity for personal use, for use as samples, for an exhibition or for use in studies, research, analysis or tests of the quality and standards
(6) the import of medical devices for particularly individual patients or animals
(7) the manufacture of medical devices as samples for export
(8) the manufacture or import of medical devices in accordance with the rules, procedures and conditions prescribed in the Notification of the Minister with the recommendation of the Commission



