Herbal Products
How to Apply for Herbal Products Permission?
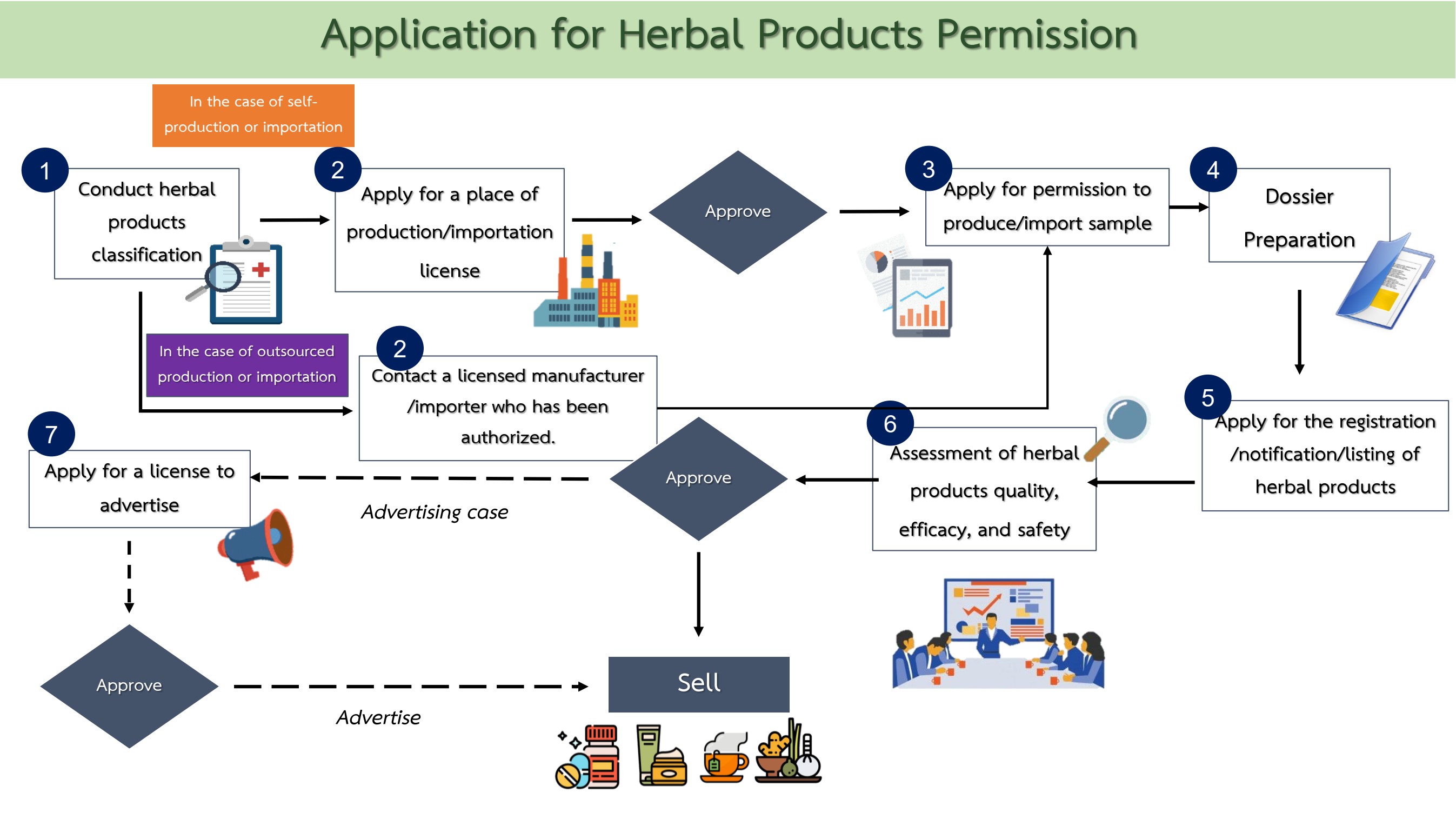
Procedural Summary
1. Product classification
In order to identify the type of herbal products, including risk assessment.
2. Application for place of production/importation
Apply for a place of producing/importing herbal products in the case where the applicant has no licensed place of production/importation, or wishes to obtain a new license.
However, the applicant can skip this process if they hire a person who is authorized to produce or import herbal products by law.
3. Application for permission to produce/import sample
Apply for permission to produce/import product sample; once granted, product sample shall be produced/imported and sent for examination or analysis as required.
4. Dossier Preparation
The applicant prepares documents for the application in accordance with the requirement of the product type.
5. Application submission
The applicant submits the application for the registration, notification or listing of herbal products to the licensing authority, as the case may be.
6. Assessment of herbal products quality, efficacy and safety
Enter the process of quality, efficacy and safety review; once permission is granted, the product may be distributed through the channels specified in the permission, which consist of “for general sale”, “for sale at licensed establishment” and “for healthcare facility only”.
7. Herbal products advertisement
If the applicant wishes to advertise the product, application for permission to advertise is required in every case.
The application process, accompanying documents and evidence required for each step by:
1. Conducting herbal products classification in order to prepare the documents and evidence correctly:
1.1 by yourself, by referring to the definition of each type of herbal products and its risk;
1.2 by using consultation e-service through the “skynet” online system; for more information, please visit One Stop Service Center
2. Applying for a place of production/importation license
By following these steps:
1) Design and apply for a planning permission (production only)
2) Request for on-site inspection (production only)
3) Learn about the application procedures at Application for a Place of Production/ Importation License
4) Submit the application
-
- Where the place of production/importation is located in Bangkok, the application may be submitted at the Food and Drug Administration;
- Where the place of production/importation is not located in Bangkok, the application may be submitted at the provincial public health office of the province in which the place of production/importation is located.
Once the license is issued, you may proceed with the application for product permission.
3. Applying for a product permission
By following these steps:
1) Learn about the application procedures at Application for Herbal Products Permission
2) Apply for a permission to produce/import product sample; once granted, product sample shall be produced/imported and sent for examination or analysis as required;
3) Submit the application in accordance with the prescribed procedures, as the case may be (listing, notification, registration), and prepare documents for the application in accordance with the product type.
Once permission is granted, the product may be distributed through the channels specified in the permission, which consist of “for general sale”, “for sale at licensed establishment” and “for healthcare facility only”.
4. Applying for a license to advertise
Follow these steps:
1) Learn about the application procedures at Application for a License to Advertise
2) Submit the application.
Once a license is issued, advertising may be carried out through the types of media permitted.
