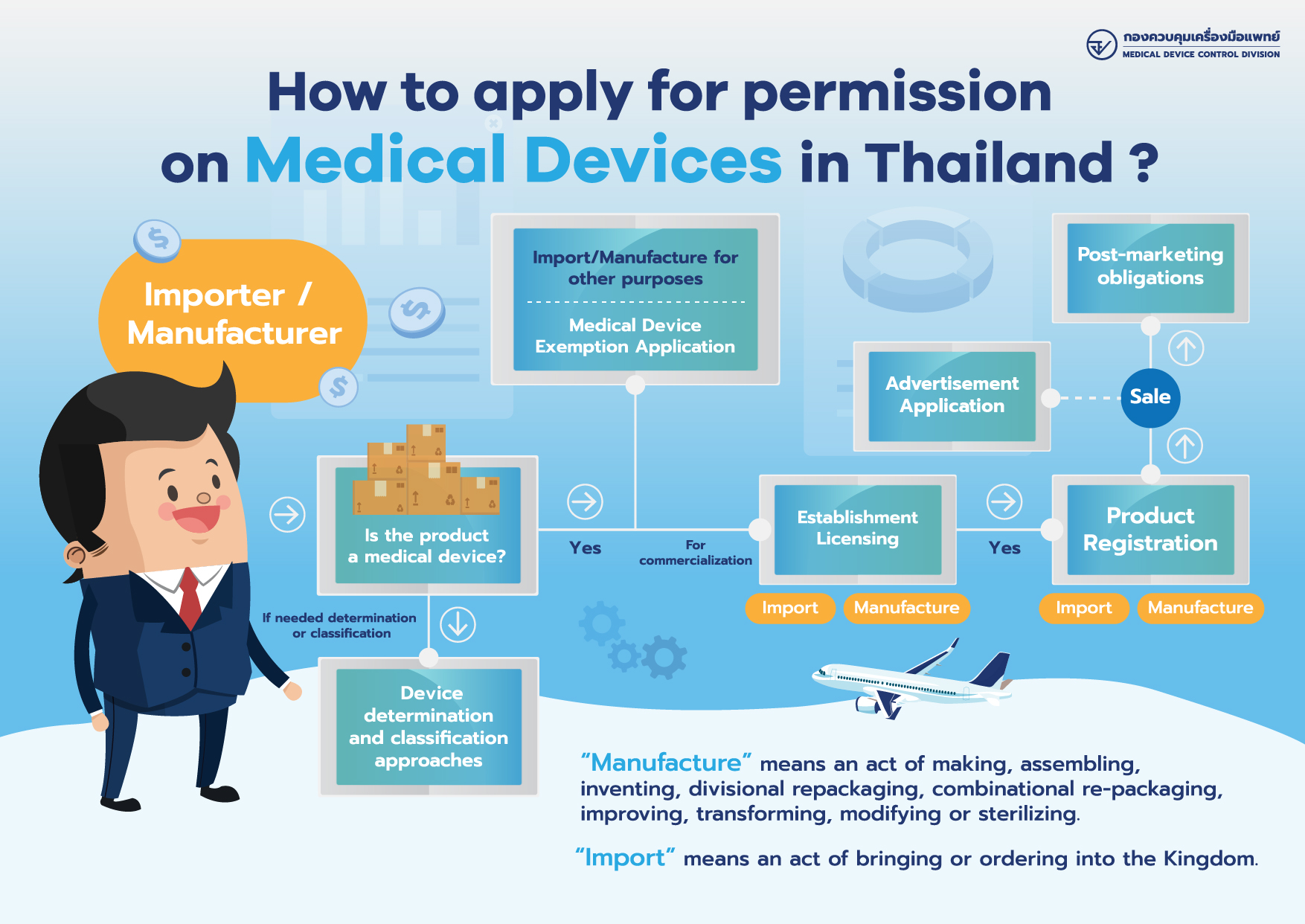
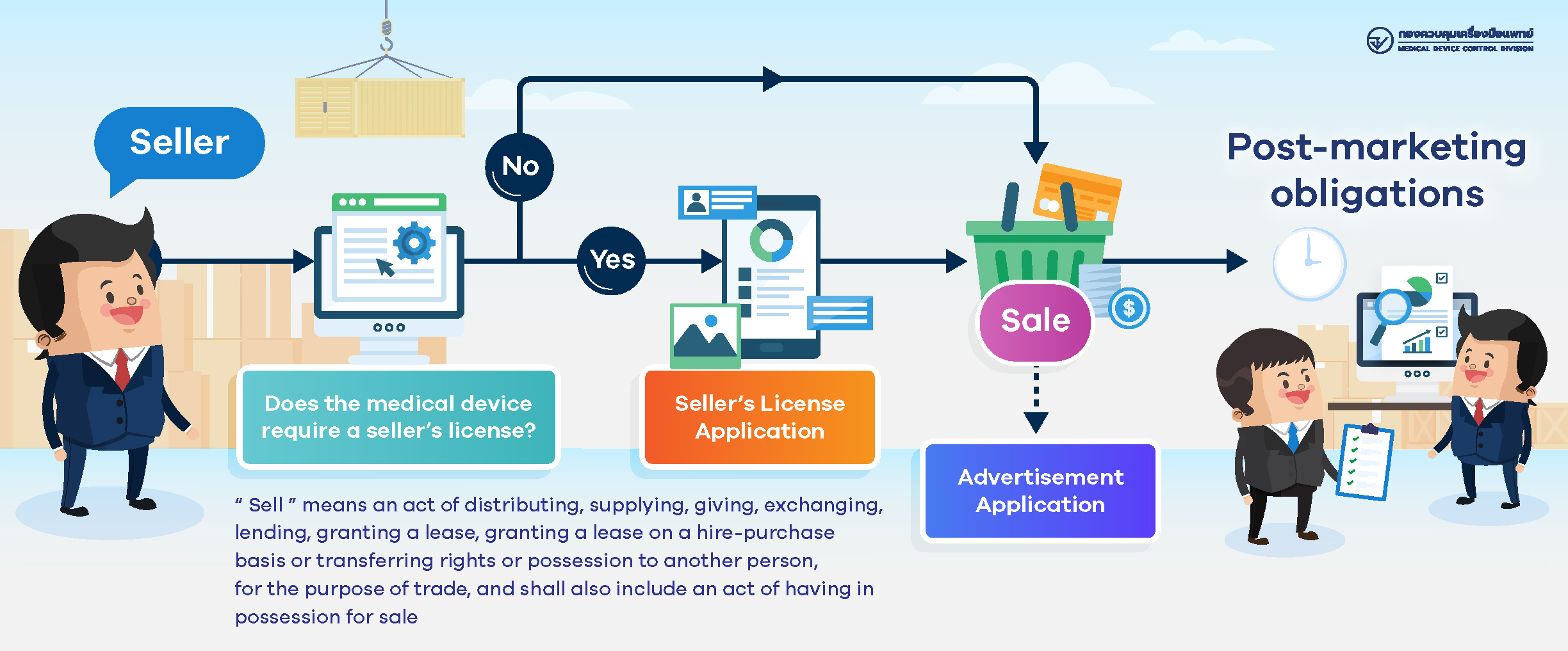
How to Apply for Permission on Medical Devices?
How to Apply for Permission on Medical Devices?
How to apply for permission on Medical Devices?
- Manufacturer/Importer of Medical Devices (Click here for more details)
- Seller of Medical Devices (Click here for more details)