One Stop Service Center
Consultation
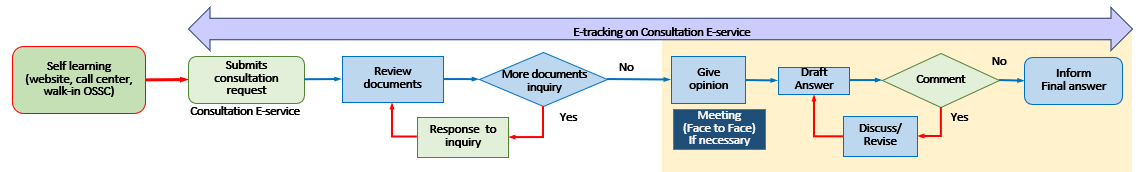
Provide consultation on academic information and regulations in relation to health products and innovations to business operators, researchers and agencies providing research funds throughout the product development process. Your representative in Thailand may request a consultation service on these topics through the
e-Consult system. Click Here. The procedure is as follows:
Scientific Advice and Protocol Assistance: provide academic consultation service by our experts in the research and development of health products in terms of their quality, efficacy and safety, such as clinical trial protocol and R&D strategy for innovative health products.
Regulatory affairs consultation: provide consultation on regulations in relation to application for permission in relation to health products.
Pre-application advice: we believe that Good Registration Management will promote efficient registration, hence we provide advisory service on preparation of documents prior to the registration process to business operators, with a view to ensuring that the preparation of documents is in accordance with the rules of the Food and Drug Administration, and promote Good Submission Practice (GSubP).
